Abstract
Introduction: Thrombosis is an important cause of death in patients with polycythemia vera (PV). The conventional two-tiered risk stratification is based on the Polycythemia Vera Study Group (PVSG) criteria and considered only age≥60 years and previous thrombosis as thrombotic risk factors. In the current study, we integrated genetic and clinical information into thrombosis risk stratification and aimed to improve the prediction power of thrombosis in the 2016 WHO-defined PV and propose risk-adapted treatment strategies according to the new model.
Methods: Patients were eligible for inclusion if 18 years or older with a confirmed diagnosis of PV according to the 2016 WHO criteria. Clinical and laboratory data were obtained from institutional databases and direct contact with patients. Bone marrow mononuclear cells were collected from the included patients and sent for next-generation sequencing (NGS). A multivariate Cox proportional hazard regression analysis was conducted to identify independent risk factors for thrombosis-free survival (TFS). Hazard ratio (HR)-based risk score allocation was employed to obtain a multifactorial prediction model.
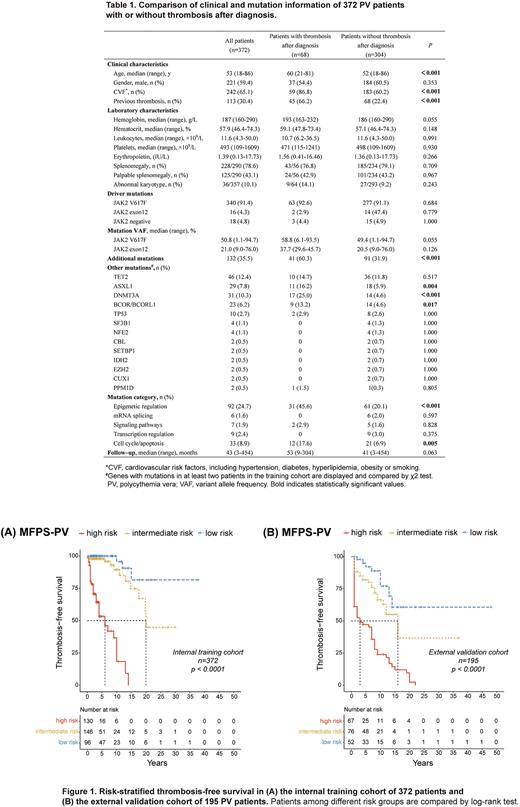
Results: The study involved 372 patients in the training cohort and another 195 patients in the external validation cohort. The median follow-up of the training cohort was 43 months (range 3-454) (Table 1). The median age at diagnosis was 53 years (range 18-86) and 59.4% were male. Altogether, 136 patients (36.6%) suffered from thrombosis, including 52 patients (14.0%) with thrombosis before diagnosis, 68 patients (18.3%) at diagnosis, and 68 patients (18.3%) after diagnosis. Mutations in genes encoding for epigenetic modifiers were more frequent in patients with thrombosis than in patients without thrombosis after diagnosis (45.6% vs. 20.1%, p<0.001). Specifically, significant differences were noted in the frequencies of ASXL1 (16.2% vs. 5.9%, p=0.004), DNMT3A (25.0% vs. 4.6%, p<0.001) and BCOR/ BCORL1 (13.2% vs. 4.6%, p=0.017) mutations between patients with and without thrombosis after diagnosis.
Multivariate analysis indicated that age≥60 years (HR 2.493, p=0.001), cardiovascular risk factors (HR 4.041, p<0.001), at least one high-risk mutation for thrombosis (mutations in DNMT3A, ASXL1, and BCOR/BCORL1) (HR 4.049, p<0.001) and previous thrombosis (HR 5.576, p<0.001) were independent risk factors for thrombosis. After assigning HR-weighted scores to each risk factor mentioned above (1, 1.5, 1.5 and 2 points respectively), a multiple factor-based prognostic score system of thrombosis was developed, classifying patients into low-risk (≤1 point), intermediate-risk (1.5-2.5 points), and high-risk (≥3 points) groups. Patients in the three groups had significantly different TFS (p<0.001) and remained consistent during external validation (Figure 1).
Finally, a risk-adapted therapeutic strategy was established based on the MFPS-PV. TP and low-dose antiplatelet therapy are recommended for all patients to achieve a targeted HCT level lower than 45%. Low-risk patients are not mandatory for cytoreduction and only require low-dose antiplatelet therapy. Intermediate-risk patients are advised for antiplatelet therapy, with cytoreduction added if they have two thrombotic risk factors, previous thrombosis, or age≥60 years. High-risk patients are recommended antiplatelet and cytoreductive combination therapy.
Conclusion: The MFPS-PV, integrating genetic and clinical characteristics for the first time, can significantly predict thrombosis and has great therapeutic implications.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal